A recent study published in the journal Redox Biology, led by researchers from the Institute of Nutrition and Food Technology (INTA) at the University of Chile, revealed a novel mechanism by which caffeic acid phenethyl ester (CAPE)—a compound found in bee-derived products like propolis—exerts its antioxidant effect in human endothelial cells. The discovery highlights the involvement of the TRPV1 ion channel in redox responses of mammalian cells, previously known for its sensitivity to compounds like capsaicin.
The research was led by Miltha Hidalgo, PhD candidate (PhD in Nutrition and Food from the University of Chile) and the young biologist Bárbara Railef, both members of the Functional Nutrition Laboratory (LINF) of INTA-University of Chile. Other researchers from INTA, Universidad Autónoma de Chile, Universidad San Sebastián and Universidad de Talca collaborated in this work.
Although CAPE has been widely recognized for its antioxidant, anti-inflammatory, and vasodilatory properties, the molecular pathway responsible for its action in human cells had remained unknown. The study titled "The antioxidant property of CAPE depends on TRPV1 channel activation in microvascular endothelial cells" demonstrated that this compound activates TRPV1, a non-selective cation channel, triggering a rapid influx of calcium ions (Ca²⁺) into the cytoplasm and an almost immediate antioxidant response in microvascular endothelial cells. (DOI: 10.1016/j.redox.2025.103507)
Professor Omar Porras, PhD in Biomedical Sciences, INTA's LINF coordinator and supervisor of the project, explains:
“Lipophilic compounds, such as fats, oils and resins, tend to interact with plasma membranes, which are lipid structures present in our cells. These membranes, composed of phospholipids, define the cell boundary. It was believed that this compound crossed the lipid bilayer by dissolving in it and acting intracellularly in a somewhat magical, nonspecific manner. However, our data indicate that caffeic acid, by possessing a phenethyl group—an additional ring that makes it hydrophobic—interacts specifically with a class of ion channels located in the plasma membrane of endothelial cells known as TRP channels. We implemented a redox biosensor in these cells, which changes its fluorescence depending on whether it is in an oxidized or reduced state. When we applied the compound extracellularly, the biosensor indicated an antioxidant effect, almost immediately.”
Physiological and therapeutic implications
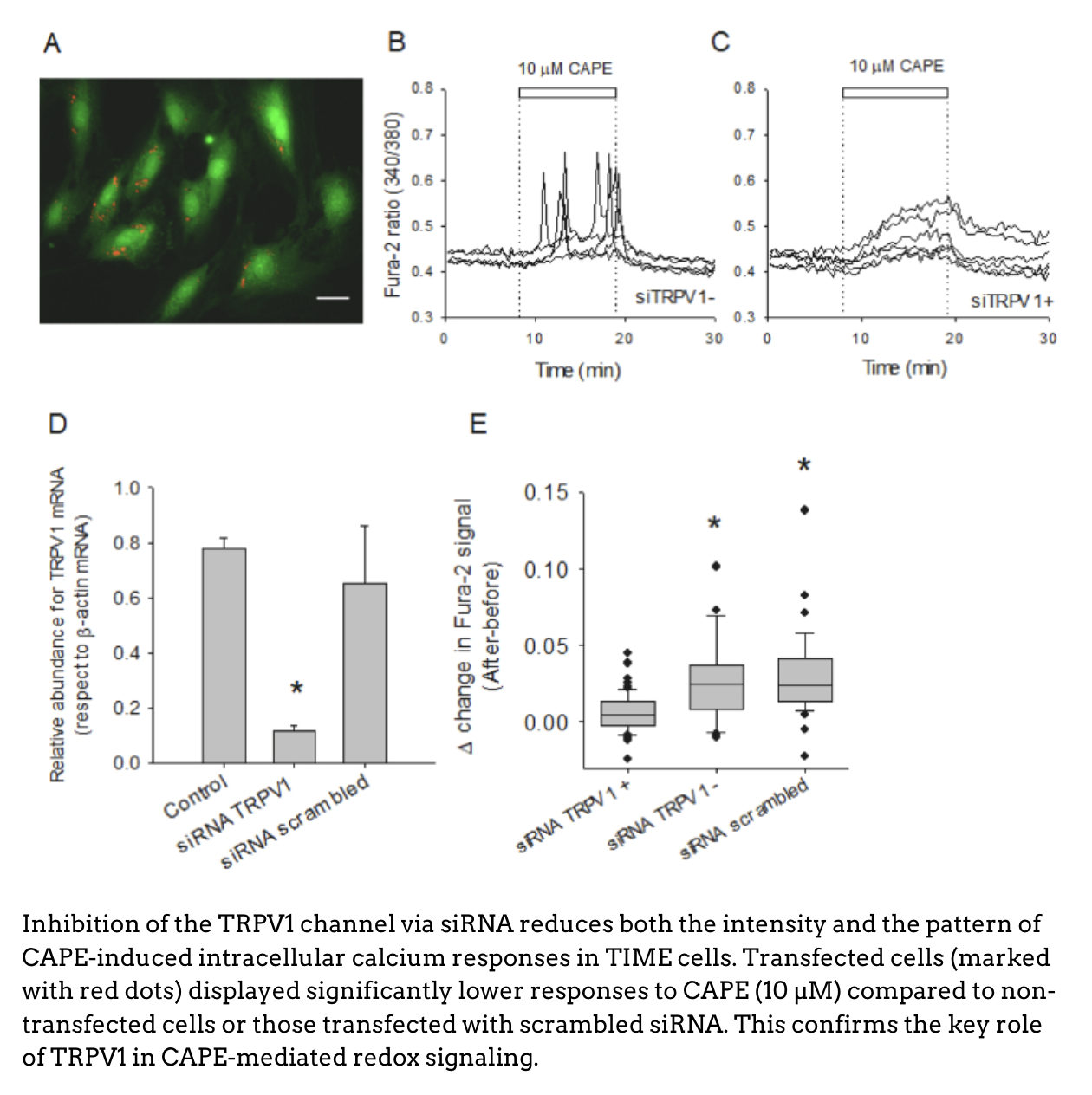
When comparing CAPE with structurally similar compounds such as caffeic acid and neochlorogenic acid, the researchers found that only CAPE triggered significant intracellular calcium responses, suggesting a specific interaction related to its greater hydrophobicity. This trait facilitates its binding to a hydrophobic pocket in the TRPV1 channel, previously described through Cryo-EM studies.
Using fluorescent biosensors (HyPer), the study showed that TRPV1 activation by CAPE is essential to trigger cytoplasmic redox changes, i.e., a real-time reduction in oxidative stress. These changes occurred within seconds to minutes, directly linking channel activation to the antioxidant effect.
These findings not only clarify the molecular mechanism of CAPE’s action, but also provide a scientific basis to understand its vasodilatory potential and therapeutic use in cardiovascular diseases. Additionally, they underscore the importance of studying how natural compounds interact with ion channels to elicit rapid and specific cellular responses.
Keywords: CAPE; Caffeic acid; TRPV1; Endothelial cells; HyPer